Choose the correct answer for each question.
1. How many atoms are there in one mole of CH3OH?
A. 6 atoms
B. 6.0 x 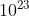 atoms
atoms
C. 12.0 x 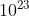 atoms
atoms
D. 3.6 x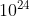 atoms
atoms
2.
Balance the equation given above. Then, find the number of moles of oxygen gas 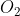 involved in the reaction.
involved in the reaction.
A. 1 mole
B. 3 moles
C. 4 moles
D. 7 moles
3. When iron pyrite ( 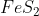 ) is heated in air, the process is known as "roasting". It forms sulfur dioxide and iron (III) oxide as follows:
) is heated in air, the process is known as "roasting". It forms sulfur dioxide and iron (III) oxide as follows:

Balance the given equation, and then, choose the correct choice of the number of moles for the reactants and products.
A. 4, 2, 8, 7 respectively
B. 2, 4, 7, 8 respectively
C. 2, 11, 7, 8 respectively
D. 4, 11, 8, 2 respectively
4.Doctors recommend taking vitamin (C) in winter days, How many moles of Vitamin C, ( 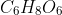 ), are there in 528 g of Vitamin C?
), are there in 528 g of Vitamin C?
given that: (C =12 , H = 1 , O = 16 )
A. 2 moles
B.2,75 moles
C.4 moles
D.5 moles
5. Which one of the following will turn red litmus solution into blue?
A. Vinegar .
B. Baking soda solution.
C. Orange juice .
D. Soft drinks .
6. Calcium is one of the most important minerals the body needs in general, and teeth in particular, because it gives the texture and the external shape of the teeth, to bear the pressure on them. Acids are the main cause of loss of the outer enamel layer of the teeth. Repeated exposure to acids causes continuous wear of the enamel over time until the tooth loses its protective layer completely and becomes vulnerable to damage. According to this, calcium phosphate ( ) which is present in tooth enamel is:
) which is present in tooth enamel is:
A.Basic
B. Amphoteric
C. Acidic
D. Neutral
7. Which acid of the following does not form an acidic salt?
A. Phosphoric acid
B. Carbonic acid
C. Hydrochloric acid
D. Sulphuric acid