9. Calculate the mass of hydrogen formed when 25 grams of aluminum reacts with an excess amount of hydrochloric acid.

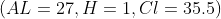
A. 0.41 g
B. 1.2 g
C. 1.8 g
D. 2.8 g
10. If 5.0 g of each reactant were used for the following process:

The limiting reactant would be:
A. 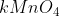
B. HCl
C. 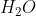
D.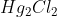
11. In the equation: 
A. 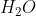 is a base and HF is its conjugate acid.
is a base and HF is its conjugate acid.
B. 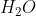 is an acid and HF is the conjugate base.
is an acid and HF is the conjugate base.
C. HF is an acid and 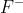 is its conjugate base.
is its conjugate base.
D. HF is a base and 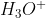 is its conjugate acid.
is its conjugate acid.